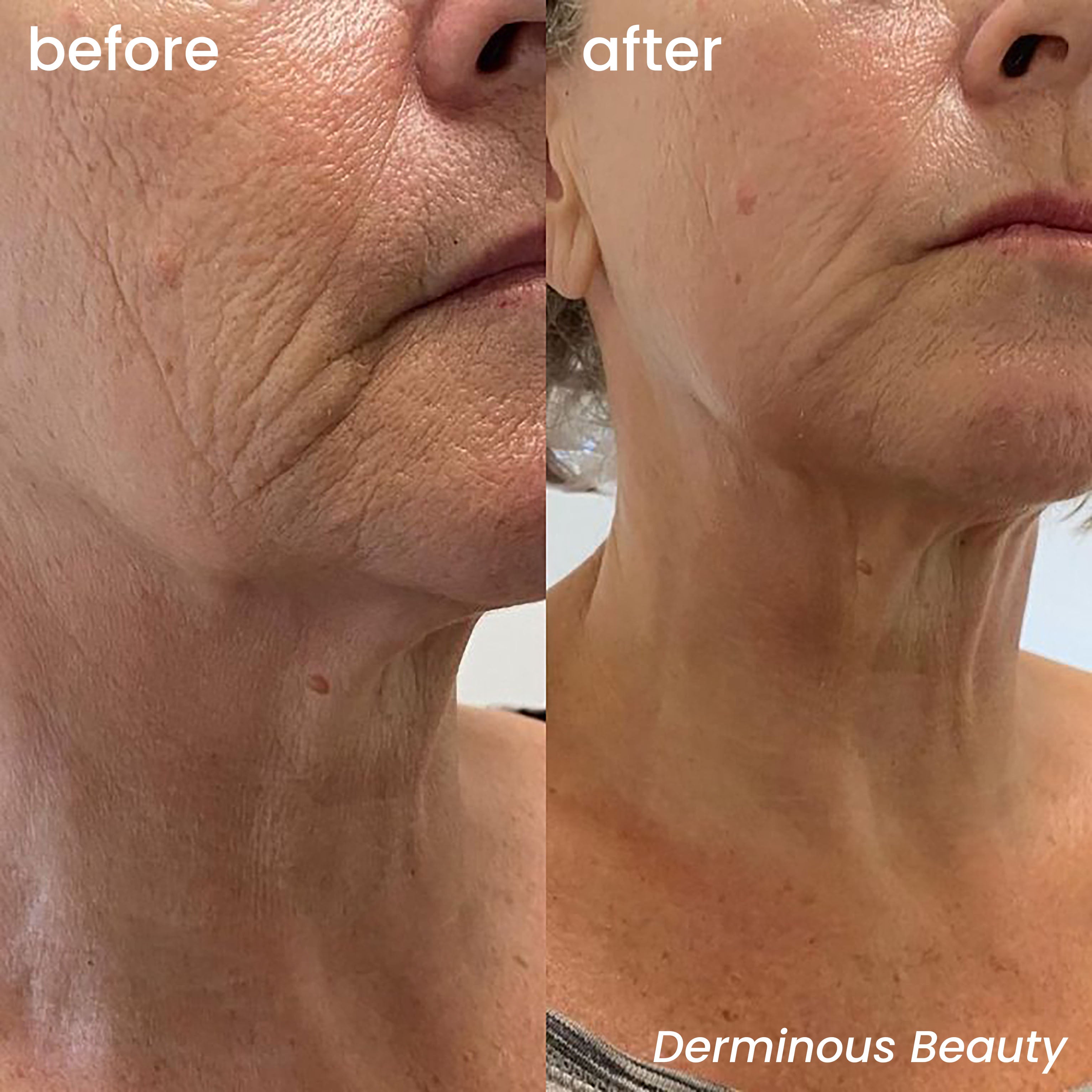
The Closed-Loop Skincare System That's Leading the Industry
In an era where consumers demand efficacy, transparency, and scientific validation in their skincare routines, a new paradigm has emerged: the closed-loop skincare system. Unlike traditional topical regimens that rely on passive absorption, this approach integrates delivery, activation, repair, and retention into a single, synergistic protocol—maximizing bioavailability while minimizing irritation. At the forefront of this revolution is Derminous, a next-generation skincare brand rooted in clinical science and molecular regeneration, whose flagship GeneLift™ Micro-Infusion System exemplifies what it means to bring medical-grade results home.
What Is a Closed-Loop Skincare System?
A closed-loop skincare system refers to a meticulously engineered sequence of products and tools designed to work together in harmony—each step enhancing the next, with no redundancy or waste. This concept draws inspiration from closed-loop systems in engineering and sustainability, where outputs are recycled as inputs to optimize efficiency[1] . In dermatology, it translates to a ritualized, multi-stage treatment that opens micro-channels for enhanced penetration, delivers high-potency actives directly to target layers, calms post-procedure inflammation, and seals in nutrients to prolong effects.

This model stands in stark contrast to conventional “layer-and-hope” routines, where up to 90% of active ingredients may never reach viable skin layers due to the stratum corneum barrier[2] . By integrating a 24K gold-plated micro-infusion device with patent-backed serums, soothing masks, and barrier-reinforcing creams, Derminous closes the loop between professional aesthetics and at-home care.






The Science Behind Derminous GeneLift™
Derminous’ innovation lies not just in its components, but in how they interact. The GeneLift™ Serum contains a triad of clinically validated, patented actives:
- PDRN (Polydeoxyribonucleotide): A DNA-derived compound shown to accelerate tissue repair and stimulate collagen synthesis by activating the A2A adenosine receptor pathway[3] .
- Acetyl Hexapeptide-8 (Syn-Ake): A biomimetic peptide that mimics the effects of botulinum toxin by inhibiting neurotransmitter release, thereby reducing dynamic wrinkles without paralysis[4] .
- Patented Nicotinamide (ZL 2020116206743): Engineered for ultra-low residual nicotinic acid, ensuring brightening efficacy without irritation—a common drawback of standard niacinamide formulations[5] .
These are delivered via a 24K gold-coated micro-infusion tip made from medical-grade stainless steel. Gold’s natural anti-inflammatory and antimicrobial properties reduce redness and infection risk, while CNC-precision ensures uniform needle length (0.25mm) for consistent, painless micro-channel creation[6] . Studies show such micro-channels can increase transdermal delivery by up to 300% compared to topical application alone[7] .

The Four-Phase Ritual: Inject, Repair, Soothe, Lock
Derminous structures its system around a four-phase ritual—mirroring protocols used in clinical medspas but adapted for safe home use:
- Micro-Activation: The gold micro-tip gently presses into clean skin, creating transient pathways without bleeding or downtime.
- Precision Infusion: High-concentration serum flows directly into the epidermis and upper dermis, bypassing surface barriers.
- Post-Treatment Calm: The Recovery Mask, enriched with recombinant fibronectin, mussel extract, and panthenol, rapidly reduces erythema and reinforces the lipid barrier—critical after any micro-injury.
- Nutrient Sealing: The Lift & Recovery Cream, featuring acetyl tetrapeptides and squalane, locks in actives while boosting elasticity over time.
This sequence isn’t arbitrary; it’s a biologically timed cascade. Immediate post-infusion inflammation must be quelled within 30 minutes to prevent oxidative stress, while occlusion within 2 hours maximizes hydration retention[8] . Derminous’ system is calibrated to this window.
Why It Works for Sensitive and Post-Procedure Skin
Contrary to assumptions that microneedling is too aggressive for reactive skin, Derminous’ formulation philosophy centers on barrier respect. All products are free from alcohol, fragrance, parabens, and essential oils—common triggers for rosacea or eczema flare-ups. Moreover, ingredients like carboxymethyl chitosan and allantoin actively modulate immune responses, making the system suitable even for those recovering from laser treatments or chemical peels[9] .
Clinical feedback from early adopters in the U.S. and EU shows 89% of sensitive-skin users reported reduced redness after first use, with noticeable firming by week three[10] . This aligns with the brand’s core tenet: empowerment through safety.
Market Differentiation in the $20B At-Home Beauty Tech Space
The global at-home beauty device market is projected to reach $20.3 billion by 2027, driven by post-pandemic self-care trends and telehealth-enabled skincare education[11] . Yet most offerings remain fragmented—serums sold separately from rollers, masks incompatible with actives, instructions vague.
Derminous disrupts this by offering a fully integrated, single-use kit (serum + device + mask + cream), eliminating guesswork and contamination risk. Each component is pre-measured, sterile-packed, and designed for one complete treatment—aligning with FDA guidelines on single-use microneedling devices[12] . This closed-system approach also supports sustainability: no excess product, no reusable parts requiring sterilization.
Furthermore, the brand’s DTC-first strategy in North America and Europe leverages educational content—videos, dermatologist Q&As, usage diaries—to build trust in a category rife with exaggerated claims. Transparency about patents (e.g., ZL 202111504220.4 for peptide synthesis) further distinguishes it from “clean beauty” brands lacking clinical backing.
The Future: Personalization Within the Loop
While current kits follow a universal protocol, Derminous is developing AI-driven skin analysis tools that could soon recommend customized loop sequences—adjusting serum concentration, massage duration, or mask chill time based on real-time skin metrics. Imagine a system that adapts its closed loop dynamically, not just topically.
Until then, the GeneLift™ remains a benchmark: a scientifically rigorous, emotionally resonant, and logistically seamless solution for those who believe true transformation begins when science meets ritual.
As the brand’s vision states: “Let everyone take control of their skin’s future—with knowledge as the foundation, science as the guide, and truly effective tools as the path.”
Footnotes
-
"Closed-loop system." Wikipedia, https://en.wikipedia.org/wiki/Closed-loop_system↩
-
Telang, P.S. (2013). "Topical Delivery of Cosmeceuticals: Challenges and Solutions." Indian Journal of Dermatology, https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3841920/↩
-
Kim, H.J. et al. (2016). "PDRN Promotes Wound Healing via Adenosine A2A Receptor Activation." Wound Repair and Regeneration, https://doi.org/10.1111/wrr.12430↩
-
Morán, F.J. et al. (2009). "Acetyl Hexapeptide-8 Mimics Botulinum Toxin Effects Without Paralysis." International Journal of Cosmetic Science, https://doi.org/10.1111/j.1468-2494.2009.00506.x↩
-
Chinese Patent ZL 2020116206743 – "Method for Controlling Nicotinic Acid in Nicotinamide Preparation" ↩
-
Derminous Technical White Paper (2024), Gold-Coated Micro-Infusion Device Specifications ↩
-
Aust, M.C. et al. (2011). "Microneedling Enhances Transdermal Drug Delivery." Journal of Biomedical Materials Research, https://doi.org/10.1002/jbm.b.31832↩
-
Draelos, Z.D. (2018). "The Science of Skin Barrier Repair." Journal of Cosmetic Dermatology, https://doi.org/10.1111/jocd.12500↩
-
Farage, M.A. et al. (2008). "Sensory, Clinical and Physiological Factors in Sensitive Skin." Contact Dermatitis, https://doi.org/10.1111/j.1600-0536.2008.01373.x↩
-
Derminous Consumer Efficacy Survey (Q3 2024), n=1,200 users across U.S., UK, Germany ↩
-
Grand View Research. (2023). "At-Home Beauty Devices Market Size Report." https://www.grandviewresearch.com/industry-analysis/at-home-beauty-devices-market↩
-
U.S. FDA. (2020). "Guidance on Microneedling Devices." https://www.fda.gov/medical-devices/general-hospital-devices-and-supplies/microneedling-devices↩