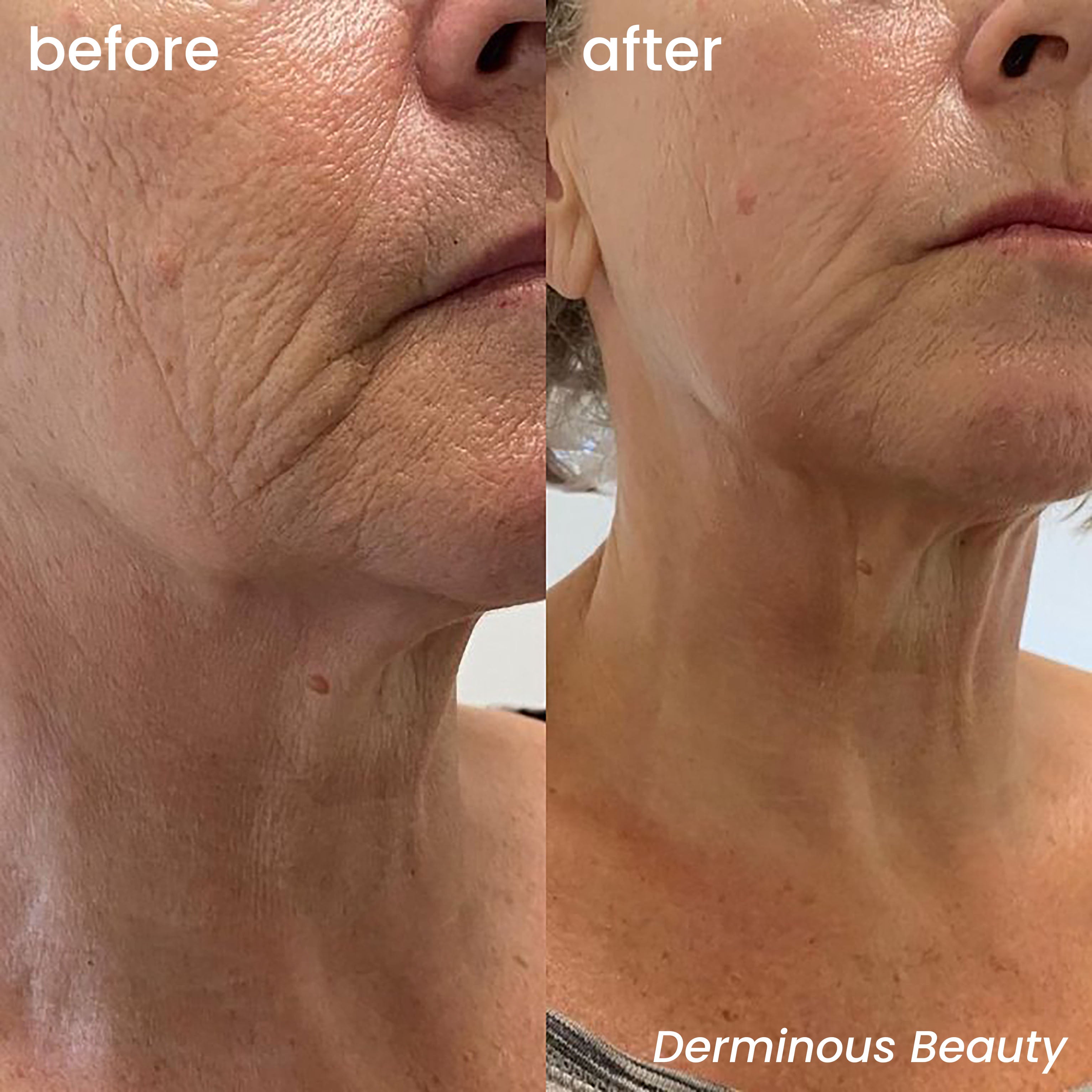
The Importance of a Sterile, Single-Use Micro-Needle Head
In the rapidly evolving world of at-home skincare technology, microneedling has emerged as a powerful method for enhancing product absorption and stimulating collagen production. However, the efficacy and safety of this treatment hinge critically on one often-overlooked component: the micro-needle head. Reusing microneedle devices—especially those not designed for multiple uses—can introduce harmful bacteria, increase infection risk, and compromise skin barrier integrity[1] .

Derminous GeneLift™ Micro-Infusion System addresses this concern head-on by incorporating sterile, single-use 24K gold-plated micro-needle heads made from medical-grade stainless steel. Gold’s natural antimicrobial properties further reduce irritation and inflammation, making it ideal for sensitive or post-procedure skin[2] . Unlike reusable derma rollers that dull over time and harbor biofilm, single-use tips ensure consistent needle length, precise microchannel creation, and optimal serum delivery—critical for activating ingredients like PDRN, peptides, and patented niacinamide formulations.

Clinical studies support that sterile, disposable microneedling tools significantly lower complication rates compared to non-sterile alternatives[3] . The U.S. Food and Drug Administration (FDA) also emphasizes the importance of single-use devices in minimizing cross-contamination in both clinical and home settings[4] .


By prioritizing sterility and disposability, Derminous not only aligns with global health standards but also empowers users to achieve professional-grade results safely—at home, without pain or downtime.





Footnotes:
Footnotes
-
Microneedling: A Comprehensive Review – Journal of Clinical and Aesthetic Dermatology. https://jcadonline.com/microneedling-a-comprehensive-review/↩
-
Antimicrobial Properties of Gold Nanoparticles – National Center for Biotechnology Information (NCBI). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6164157/↩
-
Safety and Efficacy of Microneedling Devices – Dermatologic Surgery. https://journals.lww.com/dermatologicsurgery/Abstract/2020/01000/Safety_and_Efficacy_of_Microneedling_Devices__A.10.aspx↩
-
FDA Guidance on Single-Use Medical Devices – U.S. Food and Drug Administration. https://www.fda.gov/medical-devices/reprocessing-reuse-and-single-use-devices↩